By Ross McKinnon and Talia S. Foster, M.S.
Biosimilars may offer lower-cost versions of existing biologic therapies – but a survey of Japan/Asia/Pacific (JAPAC) clinicians finds some confusion regarding differences between original biologics and biosimilars that may affect patients.
Biosimilars Resemble But Are Not Identical To Original Biologics
Generic drugs, which copy original small-molecule drugs, seek to provide exactly the same medicine at a lower price. Biosimilars, unlike generic small molecule drugs, differ from the original. For safe, effective therapy involving biosimilars, patients rely on doctors and pharmacists who may lack understanding of important issues around biosimilars.
Biosimilars may closely resemble their original biologics, but are not identical, because of differences in the way they are manufactured. Biologics are produced in living cells; the cell culture system used to produce a biosimilar, including downstream processing, usually differs from the one used to make the original biologic. Growth media used to feed the cells, and other aspects of their environment, might also vary slightly. These differences can influence a variety of factors, including safety and effectiveness.
Patients who use biologic products often have chronic diseases requiring lifelong therapy, and a longterm, stable response to that therapy. Their doctors and pharmacists serve as advisors and partners in choosing therapy that is likely to work and unlikely to cause harmful side effects. To make these decisions, clinicians must thoroughly understand the issues involved that could undermine safety. In the JAPAC region, biosimilars have become a common part of the treatment portfolio for many chronic diseases. But how well do clinicians understand the issues surrounding biosimilars?
Some Clinicians Lack Sufficient Knowledge Regarding Biosimilars
Not well, according to a survey of 670 physicians and pharmacists across JAPAC countries, including Australia, Japan, China/Hong Kong, New Zealand, Taiwan, Malaysia, South Korea, Vietnam and Singapore. Nearly half of those surveyed were rheumatologists, and the remainder were gastroenterologists, dermatologists or pharmacists. The average respondent had practiced for 18 years, mostly in a public hospital setting. On average, the surveyed physicians wrote about 20-25 prescriptions per month (81 in China), and pharmacists dispensed about 75.
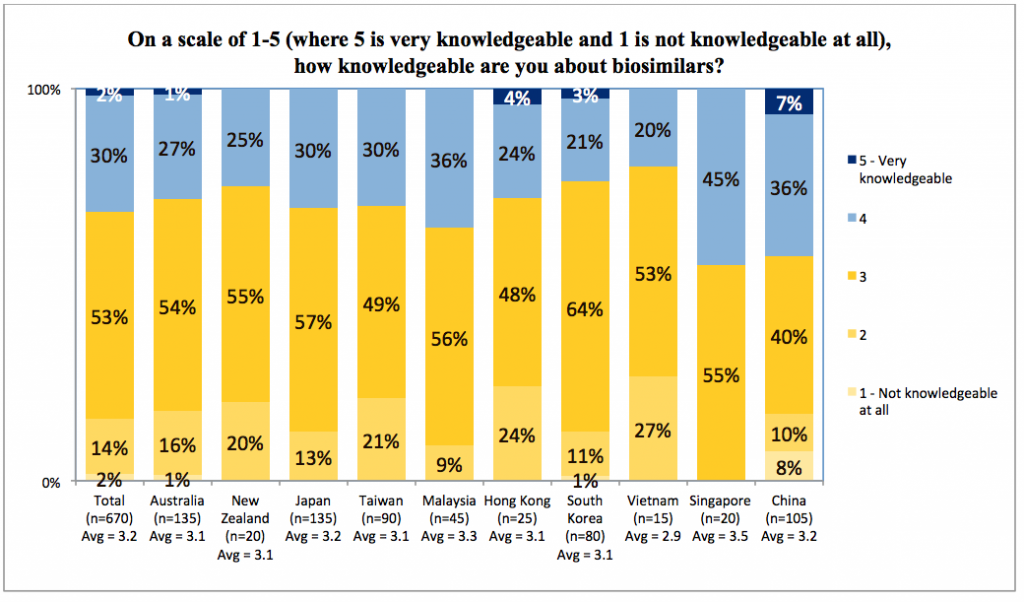
Despite their experience, two-thirds of respondents reported feeling not knowledgeable or somewhat knowledgeable about biosimilars. Fewer than five percent felt very knowledgeable. Even in China, with its high volume and longer history of biosimilar use, only seven percent felt very knowledgeable about biosimilars.
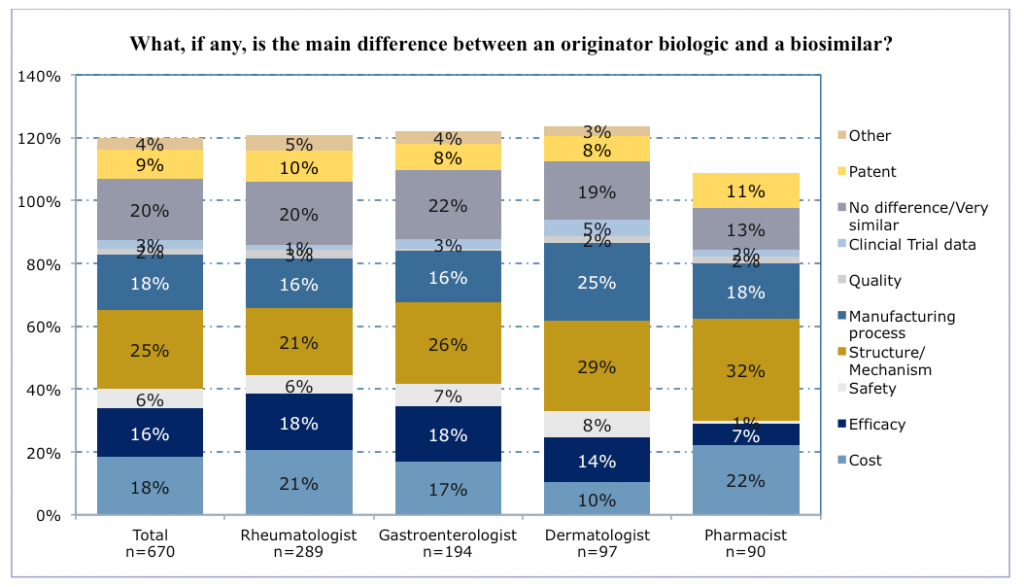
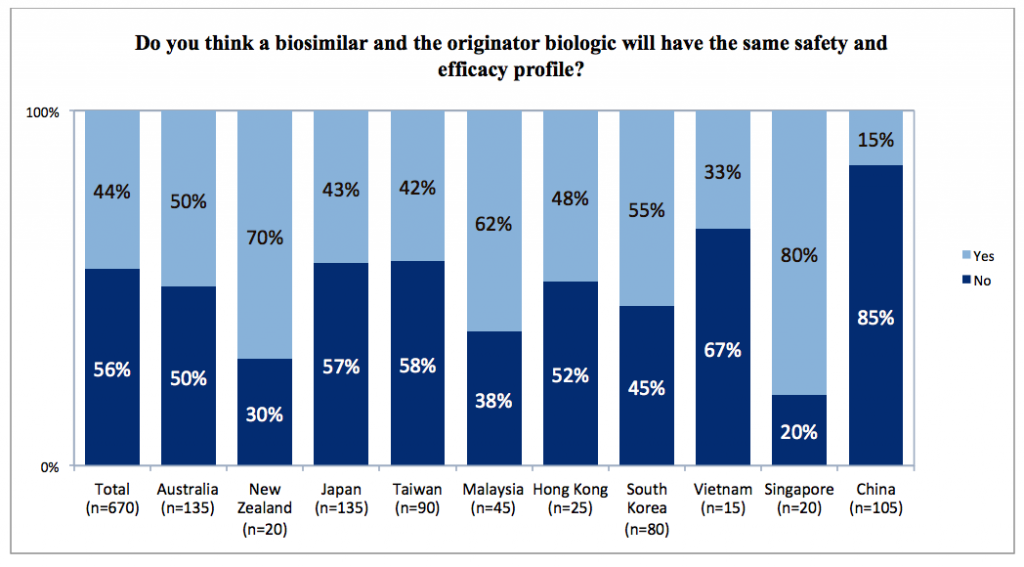
Many clinicians did recognize that biosimilars closely resembled the original biologics on which they are based but were not exact copies, and slightly more than half thought there might be differences in safety and efficacy. When asked to highlight differences, one quarter of respondents cited differences in structure and mechanism of action, and almost as many cited differences in manufacturing processes, or in their cost.
Of concern, 20% thought there was no or virtually no difference between biosimilars and original biologics. Many of these respondents also believed that clinical trials had shown their equivalence.
Physicians were much more likely than pharmacists to believe that safety and efficacy may vary between biosimilars and original biologics (22-25% vs. 8%). Dermatologists were especially likely to cite lack of evidence from clinical trials directly comparing their performance. Pharmacists, on the other hand, were more likely than physicians to emphasize cost differences (22% versus 10-21%).
Regardless of specialty, the great majority of respondents (89%) believed that biosimilars should undergo clinical studies like the original biologics to be licensed for each indication. Often they believed that this was necessary to ensure patient safety and efficacy.
Attitudes Toward Biosimilars Differ by Country
Major differences by country were clear from the survey results. Chinese clinicians were much more likely than their counterparts in other countries to believe that safety and efficacy would differ between a biosimilar and its originator biologic (85%). Indeed, they were more likely to identify safety and efficacy as the principal differences between them (63% in China versus 0-22% in other countries).
In contrast, no respondents from Singapore believed that safety and efficacy were the principal areas of difference, and 80% thought these would be the same. Singapore respondents also were much more likely than clinicians in other countries to believe that biosimilars and their originator biologics were identical or highly similar (50% in Singapore versus 9-32% in other countries).
Many Clinicians Accept Interchangeability but May Not Prescribe a Biosimilar
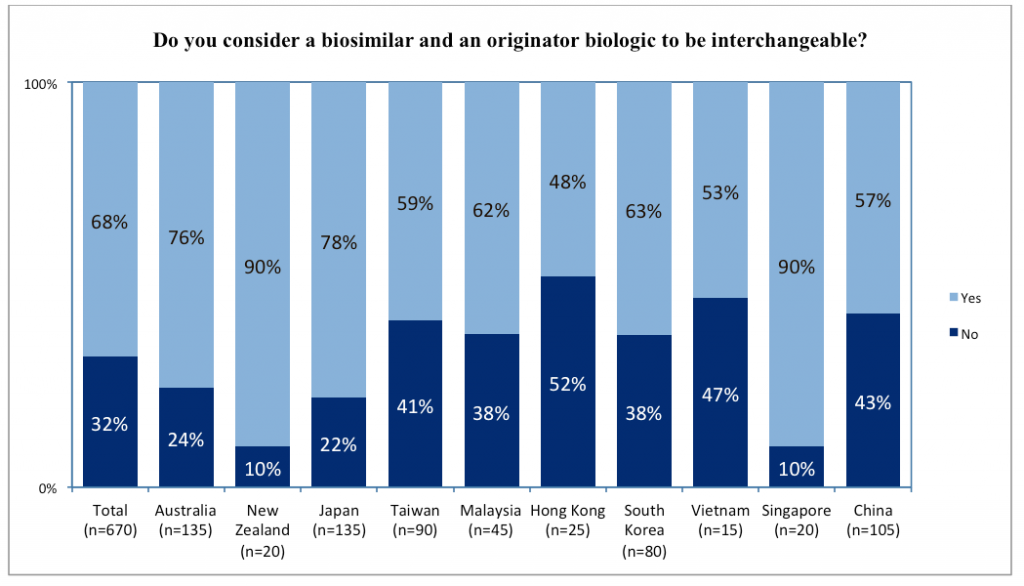
Given these viewpoints on biosimilars, how were respondents actually practising in relation to biosimilar prescribing or dispensing? Most considered biosimilars and original biologics as interchangeable. This was less true among dermatologists (55%) than other specialists (65-73%). It was also less true among respondents from Hong Kong (48%), Vietnam (53%), China (57%), and Taiwan (59%). Respondents from Singapore (90%), New Zealand (90%), Japan (78%), and Australia (76%) were much more likely to consider biosimilars interchangeable with the original biologic.
With little difference by specialty or country, respondents reported moderate approaches to the prescribing of biosimilars. Fewer than 5% believed they were very likely or very unlikely to prescribe a biosimilar. Most reported being moderately likely to prescribe a biosimilar if available, and to switch a patient from an originator biologic to a biosimilar. Pharmacists were more likely than physicians to switch, as were Chinese respondents compared to those in other countries.
While most respondents were moderately likely to prescribe a biosimilar, only a minority would start new patients on a biosimilar instead of the originator biologic. This did not vary much by specialty or country, with 37% of respondents willing to start a patient on a biosimilar.
Opinions Vary Regarding Naming of Biosimilars
When a physician writes a prescription, or a pharmacist dispenses it, the specified name of the drug may dictate what the patient takes home. While most respondents try to control this process by using both the scientific and brand names of a biologic (including a biosimilar), this practice varies by specialty and country. Pharmacists were somewhat more likely than physicians to use one or the other name rather than both. In Singapore and Malaysia, respondents were more likely to use only the brand name than to use either the scientific name or both names.
Whether the biosimilar should have the same scientific name as its originator biologic was controversial among respondents. Respondents in most countries were divided on this question. In China, respondents were much more likely to view scientific names as being very important (42% vs. 0-21% in other countries), especially for reporting adverse events.
Clinicians Want More Information About Biosimilars
Lower cost may be one of the primary reasons why health systems use biosimilars, so what leads clinicians to prescribe an original biologic instead? Many respondents viewed safety and efficacy as their primary reason for using an original biologic instead of its biosimilar. Information from clinical studies documenting such differences was also considered very important by respondents preferring an original biologic. Clinicians reported a preference for this information to be provided by various professional sources. Their first choice was conferences, followed by peer-reviewed journal articles.
Conclusions
As noted by the clinicians who responded to this survey, the potential for safety issues related to using a biosimilar instead of the original biologic underscores their need to understand the differences. Respondents did understand certain differences; they believed that biosimilars varied in structure and mechanism from originator biologics. They also thought biosimilars should be tested for safety and efficacy in each indication, just like the original biologics. Finally, they considered safety and efficacy as the primary reasons why a clinician would prescribe an original biologic instead of its biosimilar.
Despite this level of understanding, most respondents saw originator biologics and biosimilars as interchangeable. While they viewed the structure of the molecules as different, many did not view the need for different scientific names as particularly important.
Chinese clinicians use biologics much more than their counterparts in other countries, and have a longer history of using biosimilars. Perhaps as a result, they were outliers among respondents across the JAPAC region. They were more likely to switch patients to a biosimilar or start them on one, and less likely to see the need for biosimilars to have their own clinical studies. However, and somewhat in opposition to these views, they were also much more likely to see biosimilars as having different safety and efficacy as originator biologics, and did not view them as interchangeable. Any educational initiatives in China should consider the longer history and differing preferences for the use of these products in this country.
Many patients currently use biologics for long term treatment of chronic diseases, and many new biosimilars are being introduced in different markets. Therefore, the need for physicians and patients to fully understand all relevant safety considerations appears timely. Manufacturers of biologics, including biosimilars, can aid understanding by providing education to clinicians and patients. Continuing education related to biosimilars will promote the optimal use of all biologics in the patients receiving them.
Authors:
Professor Ross McKinnon
Director and Professor in Cancer Research, Flinders Centre for Innovation in Cancer
Associate Dean Research, School of Medicine, Flinders University
Vice-President, International Pharmaceutical Federation
ross.mckinnon@flinders.edu.au
www.flinders.edu.au/cancer
Talia S. Foster, M.S.
Director of Literature-Based Services, Truven Health Analytics
talia.foster@truvenhealth.com